Investors
We are an early commercial stage medtech company working to bring fully automated molecular tests to dermatology at the point of need. Dermagnostix’ product pipeline includes molecular tests for disease diagnosis, inflammatory biomarker pattern identification and prediction of treatment response.
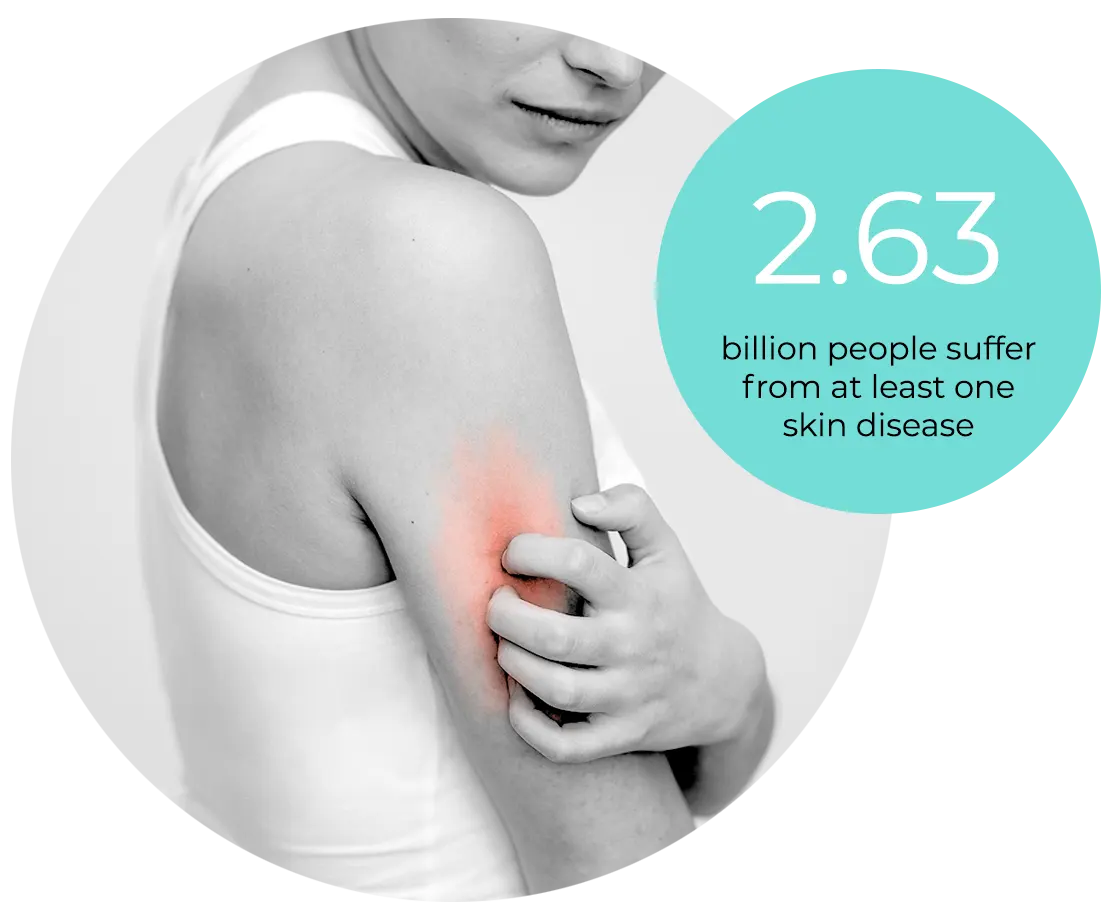
Skin health matters for human well-being
incidences of psoriasis², atopic dermatitis³ have doubled during the past decades. Often chronic, un-curable and disfiguring, skin diseases impair patients’ quality of life more drastically than cancer and diabetes⁴
’
Novel biologics and small-molecule inhibitors have revolutionized treatment options and significantly fueled dermatology drug size market which – already in 2021 - was valued at more than 26 billion US dollar⁶. Yet, diagnostics have not kept pace with therapeutic advances creating a huge gap between diagnostics and therapeutics. To fully leverage the potential of modern drugs, objective, molecular diagnostics is urgently needed to complement conventional diagnostics.
Dermagnostix offers molecular diagnostic solutions to close the gap and meet the need for personalized care.
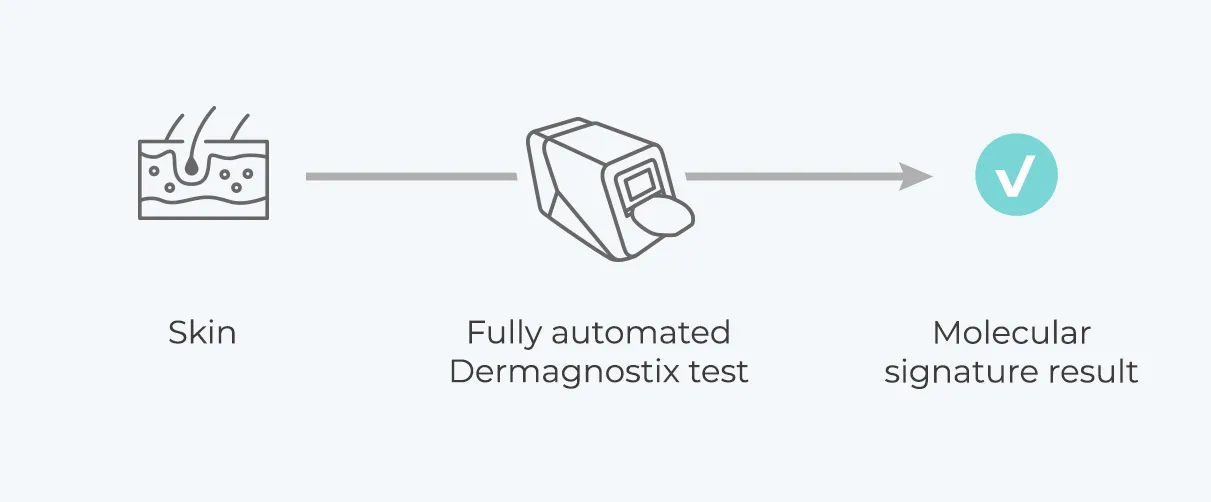
From Science to product – Dermagnostix offers comprehensive molecular diagnostic solutions
Dermagnostix provides unique diagnostic tests for unmet clinical needs in dermatology on a fully automated portable laboratory. With PsorX (CE-IVD), Dermagnostix launches the first in-class molecular test for differential diagnosis of psoriasis and eczema.
Upcoming events
Certification
Dermagnostix has been officially EN ISO 13485 certified since November 2022.
View certificate
Support programme INVEST
Dermagnostix was granted a notice of eligibility by BAFA as part of the INVEST funding measure of the Federal Ministry of Economics and Climate Protection (BMWK) to support venture capital from private investors for young innovative companies.
Find out more
Latest news
Next Generation Pathology – Dermagnostix at the DGP Annual Meeting in Munich
“We need this test in our lab!” – it’s hard to imagine a better welcome from a visitor to our booth. Even though we were serving a niche with dermatopathology at the 107th Annual Meeting of the German Society of Pathology (DGP), we were even more pleased about the enthusiasm shown by our booth visitors.
Dermagnostix expands US business development by Eureka Innowwide funding and acceptance into German Accelerator
By participating in several conferences and trade shows in the US – most recently at the ISDP meeting in San Diego – Dermagnostix has already been able to show that the interest in PsorX is high and the feedback is consistently positive. In 2024, the focus will shift even more to business development in the USA. The
References
- Flohr C, Hay R (2021): Putting the burden of skin diseases on the global map. British Journal of Dermatology 184(2): 189-190.
- Andersen LK, Davis MDP (2013): The epidemiology of skin and skin-related diseases: a review of population-based studies performed by using the Rochester Epidemiology Project. Mayo Clinic proceedings 88(12): 1462-7.
- Nutten S (2015): Atopic dermatitis: global epidemiology and risk factors. Annals of nutrition and metabolism 66 Suppl.1: 8-16.
- Montero-Vílchez T, Sánchez-Díaz M, Martínez-López A, Arias-Santiago S (2021): Quality of Life in Patients with Skin Disease and Their Cohabitants. Health-Related Quality of Life Measurement Tools, Predictors and Modifiers. IntechOpen.
- Augustin M, Glaeske G, Hagenström K (2021): Neurodermitisreport - Prävention, Versorgung und Innovation.
- Dermatology Drug Market Research | 2023-2030














